CLEVIPREX® (clevidipine) Low-volume dosing for rapid BP control

Considerations to mitigate risk of excessive fluid intake
When fluid overload may be a concern, CLEVIPREX is a low-volume dosing option for reduction of BP1
Excessive fluid intake may lead to adverse outcomes in critically ill patients2,3
The ECLIPSE trial (see safety data) was comprised of 3 parallel, prospective, randomized, open-label studies that compared the safety and efficacy of CLEVIPREX (n=752) to active comparators: nitroglycerin (n=278) and sodium nitroprusside (n=283) in the perioperative setting, or nicardipine (n=193) in the postoperative setting. Safety was the primary endpoint (defined as the incidence of death, MI, stroke, or renal dysfunction at 30 days). The study was not powered for noninferiority or superiority.4
CLEVIPREX was not studied or proven to impact fluid overload.
Low-volume and non–weight-based dosing available in ready-to-use vials1
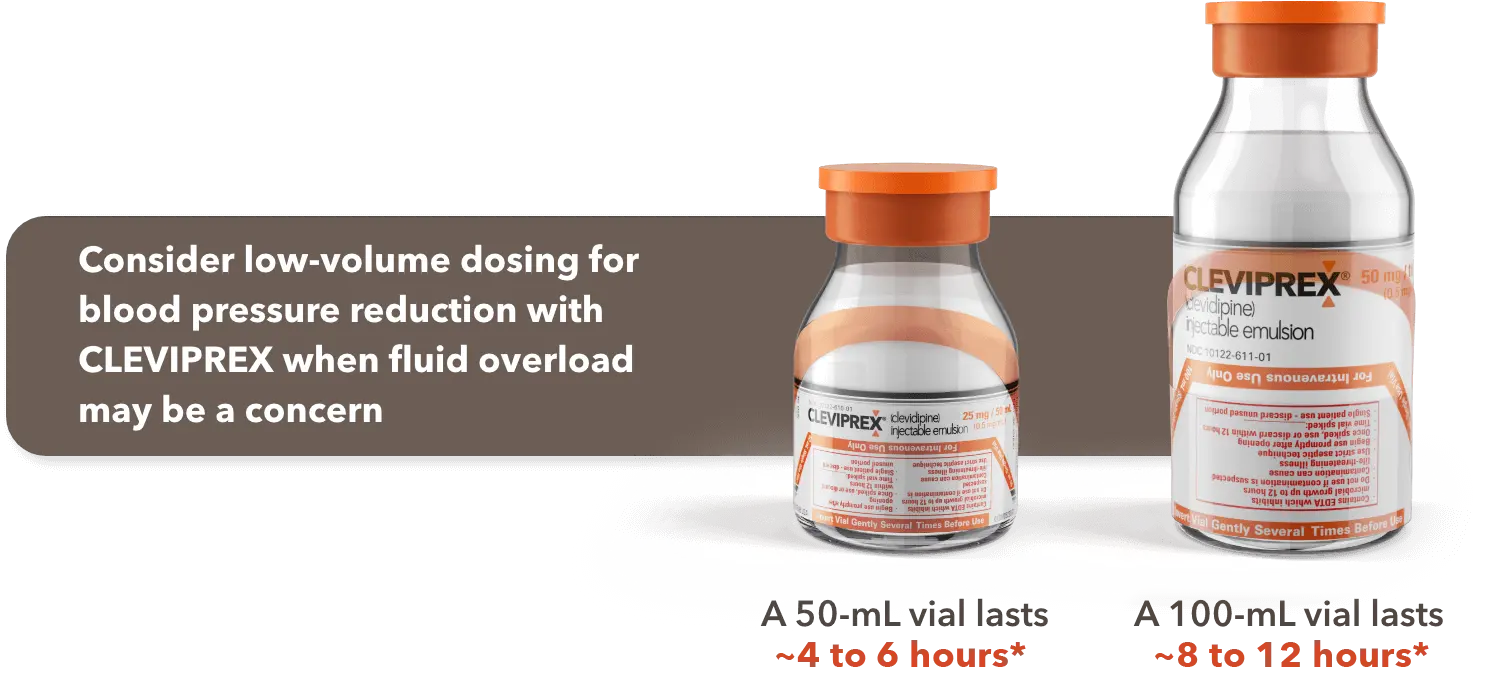
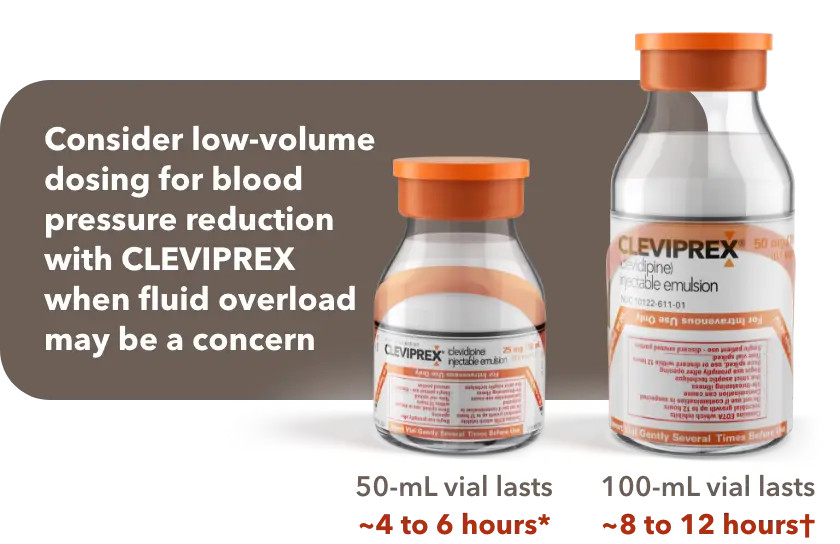
*Most patients will achieve the desired therapeutic response at approximately 4 to 6 mg/h.1
Low-volume dosing with CLEVIPREX1
Watch a video featuring Dr. Solomon Aronson, lead investigator of the ECLIPSE trials, as he discusses the trials and how CLEVIPREX was studied against active comparators. Learn how CLEVIPREX drug administration compared with nicardipine in the postoperative setting.

BP control was similar across treatments in the ECLIPSE trials4
AUC=area under the curve.
*Post hoc analysis of mITT population.
†P<0.05 vs comparator(s).
CLEVIPREX was similar to nicardipine in maintaining patients within a prespecified BP range
Efficacy was a secondary endpoint and was assessed by measuring the magnitude and duration of BP excursions above or below a predefined SBP range vs comparators.
Serious AEs were reported by day 7 or discharge4
- AEs observed within 1 hour of the end of infusion were similar between patients who received CLEVIPREX and those who received comparators1
- Incidence of AEs leading to study drug discontinuation: CLEVIPREX=5.9%; all active comparators=3.2%1
- No AEs were >2% more common with CLEVIPREX than the average of all the comparators1
Drug administration in the ECLIPSE trials4
*Based on the availability of IV nicardipine at the time of ECLIPSE, nicardipine concentration was most likely 0.1 mg/mL.
Median average infusion rate was 4x lower with CLEVIPREX than nicardipine4*
*Adapted from the ECLIPSE trial safety population data. Data presented are observational and should not be overinterpreted.
Proven efficacy in clinical studies1,5-8
CLEVIPREX provided BP reduction in a range of patients and various clinical settings.
Proven efficacy in clinical studies1,5-8
CLEVIPREX provided BP reduction in a range of patients and various clinical settings.
CLEVIPREX videos for healthcare professionals
Get access to videos about CLEVIPREX presented by renowned anesthesiologist Dr. Todd Horowitz and view other resources.
CLEVIPREX videos for healthcare professionals
Get access to videos about CLEVIPREX presented by renowned anesthesiologist Dr. Todd Horowitz and view other resources.
Transitioning off CLEVIPREX
Learn what to consider when transitioning patients off CLEVIPREX.
Treatment Transitioning
References: 1. CLEVIPREX® (clevidipine) Prescribing Information. 2021. 2. Child DL, Cao Z, Seiberlich LE, et al. The costs of fluid overload in the adult intensive care unit: is a small-volume infusion model a proactive solution? Clinicoecon Outcomes Res. 2014;7:1-8. 3. O’Connor ME, Prowle JR. Fluid overload. Crit Care Clin. 2015;31(4):803-821. 4. Aronson S, Dyke CM, Stierer KA, et al. The ECLIPSE trials: comparative studies of clevidipine to nitroglycerin, sodium nitroprusside, and nicardipine for acute hypertension treatment in cardiac surgery patients. Anesth Analg. 2008;107(4):1110-1121. 5. Levy JH, Mancao MY, Gitter R, et al. Clevidipine effectively and rapidly controls blood pressure preoperatively in cardiac surgery patients: the results of the randomized, placebo-controlled efficacy study of clevidipine assessing its preoperative antihypertensive effect in cardiac surgery-1. Anesth Analg. 2007;105(4):918-925. 6. Singla N, Warltier DC, Gandhi SD, et al; ESCAPE-2 Study Group. Treatment of acute postoperative hypertension in cardiac surgery patients: an efficacy study of clevidipine assessing its postoperative antihypertensive effect in cardiac surgery-2 (ESCAPE-2), a randomized, double-blind, placebo-controlled trial. Anesth Analg. 2008;107(1):59-67. 7. Pollack CV, Varon J, Garrison NA, Ebrahimi R, Dunbar L, Peacock WF. Clevidipine, an intravenous dihydropyridine calcium channel blocker, is safe and effective for the treatment of patients with acute severe hypertension. Ann Emerg Med. 2009;53(3):329-338. 8. Graffagnino C, Bergese S, Love J, et al. Clevidipine rapidly and safely reduces blood pressure in acute intracerebral hemorrhage: the ACCELERATE trial. Cerebrovasc Dis. 2013;36(3):173-180.
Open
Close
The information provided in this website is intended for US healthcare professionals only.
I certify that I am a US healthcare professional.